 Download this page as an activity sheet (pdf, 259KB). Download this page as an activity sheet (pdf, 259KB).
DIY DNA
In this activity, you can:
Please follow any safety instructions highlighted like this in red.
Extracting DNA from a kiwi fruit
 DNA is the polymer of life, storing the information that makes all living things what they are, from us humans to the humble kiwi fruit. It is found in the cells of every living thing, including those kiwi fruit! (If you're curious about the term polymer, you can find out more about polymers here.)
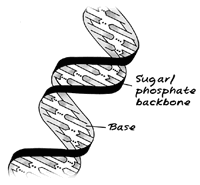 The DNA molecule has a fascinating double-helix structure, with two backbone strands joined by pairs of molecular groups called bases. Have a look at the drawing to the right, which illustrates the basic structure of DNA. It is the order of the bases that store the information necessary to describe living things – you can think of the bases as a bit like the lines of a computer program. It's difficult to imagine what a single DNA molecule looks like as it's so small. However, we can help you see a bunch of real DNA with an experiment carried out in your kitchen to extract the DNA from a kiwi fruit.
Materials – what you'll need
- A bottle of methylated spirits,
- some salt,
- cheap washing-up liquid (concentrated doesn't work as well),
- kiwi fruit (preferably ripe),
- some ice,
- some hot water,
- a sharp knife and chopping board,
- kitchen scales,
- a measuring jug,
- two bowls (one small, one large),
- a saucepan,
- a fork,
- a sieve and
- a glass.
Method – what you need to do
- Put the methylated spirits into a large bowl of ice so it cools straight away (see Figure 1). Do not put methylated spirits into a fridge or freezer as a spark could ignite fumes.
- Mix of 25g salt and 80g of washing-up liquid with 900ml water in a small bowl. Stir carefully to avoid too much froth (Figures 2a and 2b).
Figure 1.Figure 2a.Figure 2b.
  
- Peel a kiwi fruit and chop finely. Using a fork, mash the kiwi fruit
into a paste (Figure 3).
- Put the kiwi paste into a small bowl and add 100ml of the salt-detergent
mix from step 2. Sit this in a saucepan of hot (not boiling) water for 15 minutes (Figures 4a and b).
Figure 3.Figure 4a.Figure 4b.
  
- Pour the green paste through the sieve into a glass (Figure 5).
- Drizzle the ice cold methylated spirits down the side of the glass so it
forms a purple layer on top of the green kiwi paste. You will need an equal amount
of methylated spirits and kiwi paste (Figure 6).
- You should see a white string-like layer form in the glass between the green and the
purple layers. This is your extracted kiwi fruit DNA – congratulations! (Figure 7).
Figure 5.Figure 6.Figure 7.
  
How does the DNA extraction work? – the science bit
By chopping and mashing up the kiwi fruit, then leaving it in the salt and
detergent mix, we break open the cell walls, called membranes. This lets all the cell
contents out, including the DNA. But the DNA is still surrounded by polymers called
proteins. Luckily, kiwi fruit contain an enzyme called proteinase – this attacks and
breaks up the proteins, freeing the DNA.
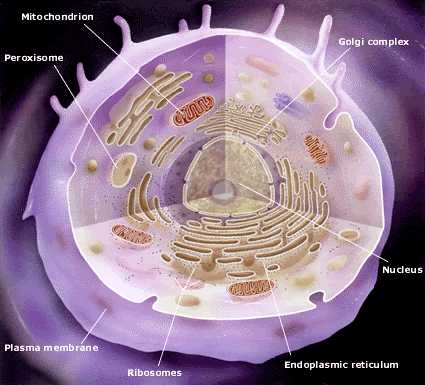 DNA is found within the chromosomes inside the nucleus of the cells that make up every living thing, including your kiwi fruit. To extract this DNA, we have to separate it from all the other cell parts. This picture illustrates a cross-section through a cell – the DNA we want to extract can be found in the nucleus, at the very heart of the cell.
The green kiwi paste now contains your freed DNA, but also has all the other cell stuff that you have released. Passing it through a sieve removes most of these unwanted bits. Then, when you pour the methylated spirits on top, the DNA turns into a solid, because it can't stay dissolved in the methylated spirits.
You might get bubbles in between the purple and green layers. This is because of
the different temperatures of the two layers. It makes the air dissolved in the green layer come out as bubbles!
This experiment relies on an enzyme in the kiwi fruit to unlock the DNA. Enzymes are powerful polymer machines that help make things work faster (you can find out more about enzymes below). Apples and oranges don't have enough of these enzymes to work with this experiment, as the DNA won't be set free. However, an onion does!
Back to the top.
How do enzymes work?
Enzymes catalyse (speed up) reactions in living things – they oil the wheels of the machinery of life! Each enzyme operates on a specific substance, called its substrate. They fit together like a lock and key, allowing only the correct combination.
Have a look at our diagram below. It shows an enzyme (E) catalysing the breakdown of another substance (S, the substrate) into two different products (P). Catalysis occurs because substance S fits precisely into surface of the enzyme E, so this reaction and no others are speeded up.
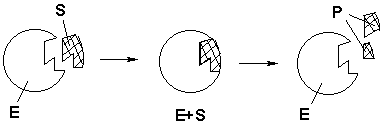
Here are two simple experiments to show enzymes in action. You'll need:
- a ripe fresh pineapple,
- a tin of pineapple,
- fruit jelly cubes (or another gelatin product),
- 3 small bowls and a knife.
First experiment – fresh pineapple
- Prepare the jelly according to the instructions on the packet.
- Leave it to cool and set in a small bowl.
- Once the jelly has set, cut a piece off the fresh pineapple and place it on top.
- Observe and record results.
Second experiment – fresh and tinned pineapple
- Prepare enough jelly for two bowls, following the packet instructions.
- Leave to set in two small bowls, one with a piece of tinned pineapple in, the other with pieces of the fresh pineapple in.
- Observe and record results.
What's going on? – the science bit
There are many types of proteins, all made up of units called amino acids. Altogether there are 20 amino acids. They are linked in chains of different lengths and orders to make all the different proteins. Gelatin is a protein, which causes the jelly to set; enzymes are a special type of protein too and can be found in the pineapple.
In the first experiment, when you place a chunk of fresh pineapple on top of the jelly, enzymes from the pineapple catalyse the digestion of the gelatin in the jelly. Therefore, the chunk of pineapple starts to sink into the jelly as its enyzmes help breakdown the gelatin.
In the second experiment, the jelly containing the tinned pineapple
should set much more effectively than the jelly with the fresh pineapple. Enzymes from the fresh pineapple are breaking down the gelatin that the jelly needs to set properly. With the tinned pineapple, this doesn't seem to be a problem. Can you think why?
It turns out that enzyme molecules are sensitive to heat – their unique structures are destroyed by heat and they are no longer able to function as catalysts. Most tinned foods are heat-treated during pasteurisation to prolong their shelf life. In contrast to the fresh pineapple, the enzymes in the tinned pineapple have been denatured (destroyed) by heat and can't assist in the digestion of the gelatin protein. | 
