A Single Crystalline Gold Acetylene Complex is “Hot”
Posted on Wednesday 10 July 2024
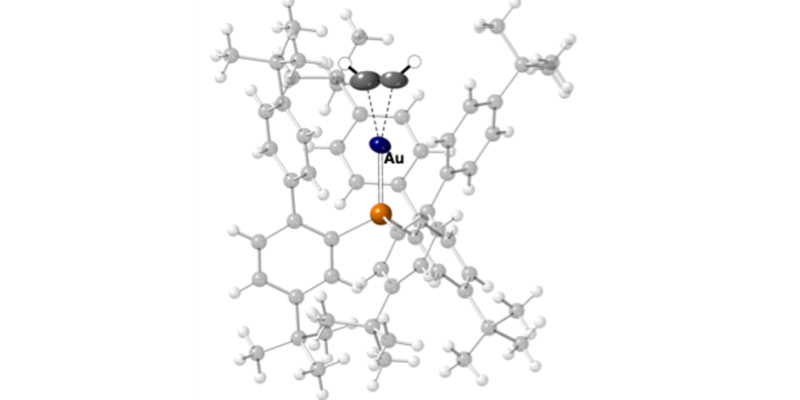
Cationic gold-phosphine complexes are powerful catalysts for the functionalisation of alkynes in organic synthesis. The key intermediates in cationic gold-phosphine catalysis are complexes in which the alkyne coordinates to the metal centre, to form a so-called π-complex. Acetylene, HC≡CH, is the simplest alkyne and an important C2 chemical feedstock, but π-complexes with gold are unknown. This is because, in solution, such complexes are very reactive and decompose, meaning that – until now – their characterisation and isolation has not been possible.
In a recent paper published as a “Hot Article” in Angew. Chem. Int. Ed., coming from a project funded by the Leverhulme Trust, York PhD student Chloe Johnson (Duckett and Weller groups) has shown that a stable gold-acetylene π-complex can be made using single-crystal to single-crystal techniques, in a method that completely avoids the use of solvent. Addition of gaseous HC≡CH to a phosphine gold-carbon monoxide precursor directly in the single-crystal results in the clean formation of a new complex in which acetylene is shown to bind to the Au-centre using its π-bond.
Commenting on the discovery, Professor Andrew Weller said: “Chloe’s paper showcases the power of single-crystal organometallic reactivity, and an exciting opportunity we are currently exploring is the using this, and other, systems for precisely-defined, very efficient, single-crystal molecular catalysis.”
This synthesis was made possible through a combination of the single-crystal methods developed in the Weller group and the cavity-like phosphine ligand developed by Jesús Campos and his group in Seville. The bonding in the new complex was analysed by Professor Stuart Macgregor and his team at St Andrews. The key synthetic steps were carried out on a visit to Seville by Chloe, on a collaborative trip generously funded by the Wild Overseas Scholars Fund.
First author on the paper, Chloe Johnson, said: “The opportunity to travel internationally to another centre of excellence made this work possible, and I encourage any Chemistry PhD students at York to take advantage of our unique and generous Wild Scholars’ fund to support their own research.”

Chloe and postdoctoral researcher Miquel Navarro during her visit to Seville.
This research was recently published in Angewandte Chemie International Edition.
