Dr Michael Plevin
Senior Lecturer and Royal Society Industry Fellow
Research
My research addresses mechanisms of biomolecular recognition and the structural and chemical features that define interaction surfaces of proteins and nucleic acids. Within this broad subject area, I have a particular interest in molecular recognition by multi-domain proteins, considering both the interactions mediated by structured domains and the roles of the flexible regions that link these domains.
A major research focus is the design and engineering of proteins for industrial applications. We use our knowledge of protein structure and function and expertise in structural biology, biophysics and biochemistry to generate proteins that address industrial questions. We combine rational, phage display and computational approaches to generate synthetic proteins. Current projects in this area involve development of novel non-antibody scaffolds, recombinant protein tags and DNA helicases and nanopores. Our work is funded through UKRI, The Royal Society, CASE studentships and direct industry funding. Current partners include Eluceda Ltd, Syngenta and Oxford Nanopore Technologies.
Figure 1. We use structural biology tools including X-ray crystallography, single particle cryoEM and NMR spectroscopy to characterise and engineer recombinant proteins.
Liquid-liquid phase separation (LLPS) is a fascinating area of biophysical science that sits nicely at the interface of our interests in non-covalent interactions and multi-domain proteins. My group has an interest in this phenomenon, particularly the role of modular proteins, intrinsic disorder and weak transient interactions in the process of phase separation, how phase separation underpins the emergence of organization and function in the cell, and the use of solution biophysical techniques to characterize these processes. We collaborate with several groups in this area, including examining the biophysical drivers and functional consequences of phase separation of Rubisco in marine algae, which we do in collaboration with Luke Mackinder (algal molecular biology) and Mark Leake (single molecule biophysics).
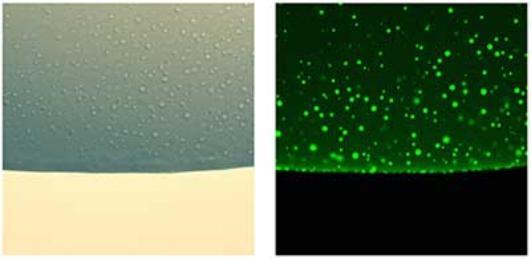
Figure 2. Studying biomolecular phase separation in vitro using recombinant proteins and light and fluorescence microscopy.
Another research theme relates to non-covalent interactions involving aromatic groups in biomacromolecules. We have developed approaches for detecting aromatic hydrogen-bond-like interactions in proteins and determined their contribution to protein-protein recognition. These studies provided the first definitive experimental evidence for the existence of two types of XH/pi interaction in proteins. Our current work investigates how these weak interactions contribute to the structure, dynamics and function of proteins. We work with Jon Agirre in York to examine how effectively these interactions are captured in experimental and predicted protein structures.

Figure 3. XH/pi interactions in proteins: Tyrosine-59 and Leucine-50 in human ubiquitin (1UBQ)
I believe strongly in promoting positive research culture and am a founding member of the Enhancing Research Culture working group. You can learn more about our activities via our webpage:
Teaching and scholarship
![]()
My teaching at York is heavily influenced by the activities of my research group, the biological processes we study, and the techniques we use.
![]()
I contribute to a Stage 2 module that introduces methods for elucidating the 3D structures of proteins and other biomacromolecules, in which I focus on solution-based biophysical techniques such as solution-state NMR spectroscopy.
![]()
I run a Stage 3 group project on protein engineering for Integrated Masters students. Students are tasked with designing, producing and characterising a synthetic protein. The project links computational and bioinformatic tools with recombinant expression and in vitro characterisation of the proteins that the students have designed.
The Stage 4 projects available in my research group align closely with our research activities. Themes of protein engineering, protein modularity, molecular recognition, sequence-structure-function relationships will be central to any project we supervise. We aim to train project students in advanced laboratory techniques, including recombinant protein expression and purification, biophysical analysis of protein stability, analyses of protein/protein interactions, the use of NMR spectroscopy, protein crystallography or cryoEM for studying protein structure, as well as computational tools for predicting, designing and analysing protein structures.



