Force-Induced Amyloids in Fungal Biofilms: Adhesion, Catch Bonding, and Host Response
Thursday 1 February 2018, 4.00PM
Speaker(s): Peter Lipke, Biology Department, Brooklyn College of the City University of New York
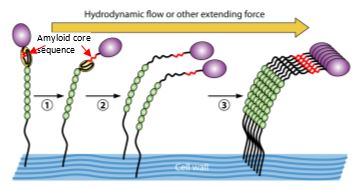
Fungal adhesins are 1200-1500 amino acids long, and are anchored to cell wall glucan. Almost all of these proteins include amyloid core sequences of 5-7 amino acids which mediate amyloid fiber formation in soluble forms of the adhesins. These same sequences also cluster wall-bound adhesins into 100-500 nm-diameter amyloid nanodomains in vivo on the cell surface. Formation of these adhesin nanodomains is abrogated in mutants without an amyloid sequence, and is inhibited in the presence of anti-amyloid compounds. Many of the amyloid-like properties of Candida albicans Als5p are preserved when its native amyloid core sequence is replaced with an amyloid core sequence from the human Alzheimer’s disease protein Ab.
Consequences of nanodomain formation include increased strength of adhesion due to high avidity binding of the clustered adhesins, as well as apparent amyloid formation in trans between cells. AFM and laminar flow experiments with cells expressing the adhesin CaAls5p or ScFlo1p or ScFlo11p show that shear stresses of 0.5 dynes/sq. cm2 or greater expose the amyloid core sequences, and cell surface adhesin nanodomains then form on the cell surface within a few minutes. As a consequence, fungi expressing amyloid-forming adhesins show catch-bonding under flow. This flow-dependent catch-bonding leads to increased biofilm mass. As expected from these results, there is amyloid on the surface of C. albicans, C. parapsilosis, C. tropicalis and S. cerevisiae in biofilms. There are also medical consequences in diseased tissue from victims of deep mycoses including candidiasis, aspergillosis, coccidioidomycosis, and mucormycosis. The cell walls of the fungi are coated with amyloid, which binds the amyloid-PRR Serum Amyloid P component (SAP). SAP binding downregulates phagocytosis by macrophages and may allow immune evasion. Therefore, cell wall amyloid nanodomains promote both fungal biofilm formation and affect host responses to fungal infections.
Location: B/M/052
Email: jennifer.potts@york.ac.uk