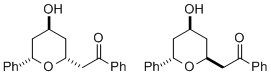Current Targets for Total Synthesis
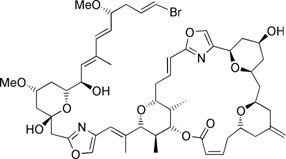
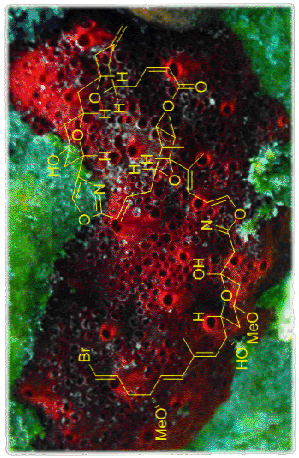
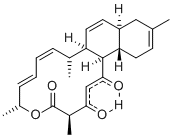
(+)-Phorboxazole B: Is a potent anticancer agent which is no longer available from nature. Our highly convergent approach uses our recent development of the asymmetric Maitland-Japp pyran forming reaction and a novel stereodivergent Michael reaction to rapidly assemble the tetrahydropyran units present. This will enable the individual pyran fragments to be coupled efficiently, minimising the need for further manipulations.
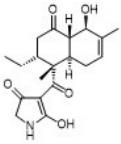
Streptosetin A: Is a recently isolated marine-derived actinomycetes which exhibits human class III HDAC (SIRT) inhibition. This is interesting as other inhibitors demonstrate apoptotic and autophagic cell death in breast cancer cell lines. We are progressing a total synthesis of this molecule due to its biological activity and its interesting decalin and tetramic acid structural features.
Completed Natural Products
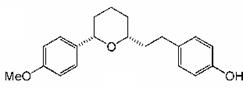
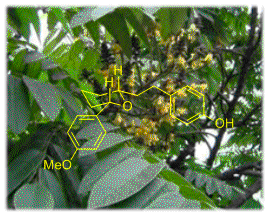
(±)-Centrolobine: Use of a 3 component, one pot reaction for the formation of highly substituted tetrahydropyrans has enabled us to synthesise the antibiotic molecule centrolobine in only 4 steps.
(±)-Civet Cat Secretion: Use of the dihydropyran version of the Maitland-Japp reaction has enabled the diastereoselective synthesis of the molecule found in Civet cat secretion.
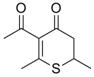
(±)-Citreothiopyrane A: Use of a thiopyranone modification of the Maitland-Japp reaction has led to the synthesis of this metabolite of penicillium citreo-viride B. IFO 6200 and 4692, a natural product with plant growth inhibition activity.
New Methodologies
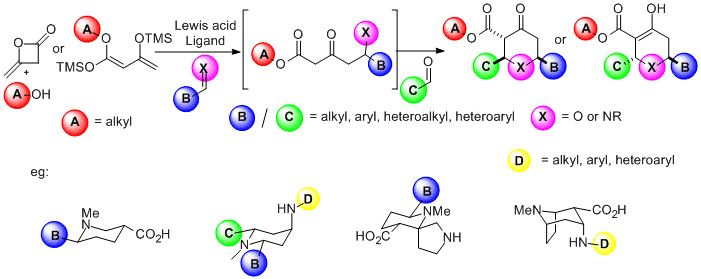
PASE synthesis: We have developed a new pot, atom and step economic (PASE) synthesis of highly substituted tetrahydropyranones, which does not involve a Diels-Alder cyclisation. Our work has delivered two enantioselective versions of this reaction, which generate THPs in 99%e.e. We are now focused on expanding the utility of this reaction to enable the asymmetric synthesis of piperidine rings and structurally diverse 3D-heterocyclic fragments with are of substantial interest to medicinal chemists for the development of new drug molecules.
Prebiotic Chemistry
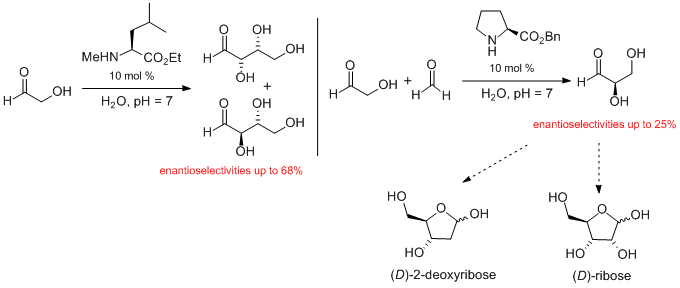
A fundamental question of science is how did Life arise on the early Earth? Obviously, for Life to arise all of its building blocks must have been in place. However, there is intense debate on how the essential building blocks of Life, such as carbohydrates, inorganic phosphate and simple cells formed in the absence of any biological processes. The group applies synthetic chemistry techniques to understand these processes. To date we have shown that amino esters and amino nitriles (precursors of amino acids) can promote the formation of glyceraldehyde, threose, erythrose and 2-deoxy-D-ribose with the highest enantioselectivities reported.
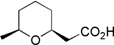
(±)-Diospongin A and B: Application of our stereodivergent oxy-Michael reaction has furnished efficent syntheses of both natural products from a single precursor.
Anthracimycin: With the dire need for new antibiotics being constantly highlighted by both government and health professionals we have initiated studies directed at completing the first total synthesis of anthracimycin. This recently isolated marine natural product has potent antibacterial activity and an unknown mode of action. We aim to produce quantities of the natural product and strutural analogues in order to probe anthracimycin's mode of action and determine whether it has the potential to be a new weapon in the war against antibiotic resistance "superbugs".

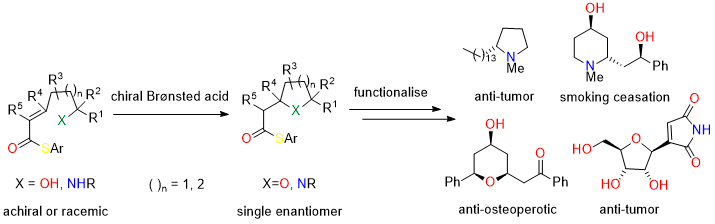
Asymmetric Heterocyclisations: Cyclic amines and ethers are important structural units present in many pharmaceutical agents and natural products. The ability to synthesise these units with control of both relative and absolute stereiochemistry is becoming increasingly important. In this project we are developing the asymmetric oxa- and aza-Michael reactions of w-substituted unsaturated thioesters for the synthesis of pyrrolidines, piperidines, THFs and THPs from both achiral and chiral racemic precursors. These methods will be applied to the synthesis of both polyketide and alkaloid containing natural products.